Tackling emissions in aluminium production
.webp)
Alongside steel, aluminium is the most important metal that drives much of the core economy, such as the automotive sector, construction, packaging and many other vital industries that keep our daily lives on track. The fact that there has hardly been a year in the last 20 years without an increase in production underlines its continuing importance (Lightmetalage.com).
As is the case with major metals, they come with a hefty carbon footprint. Three percent of global emissions come from aluminium production, and the annual decline in emissions is half of what is needed to reach the net zero scenario for 2050, according to the IEA. (Tracking Aluminum,2023) Aluminium has a unique quality though: Theoretically it can be recycled again and again without quality loss. And producing recycled or secondary aluminium demands a fraction of the energy and carbon emissions the primary production route demands.
The energy requirement for primary aluminium is 15.7 MWh per tonne, while recycling requires only 0.8 MWh per tonne. Emissions are also a fraction of the 16t CO2 per ton of primary aluminium, at 0.5t CO2 per ton of recycled aluminium.
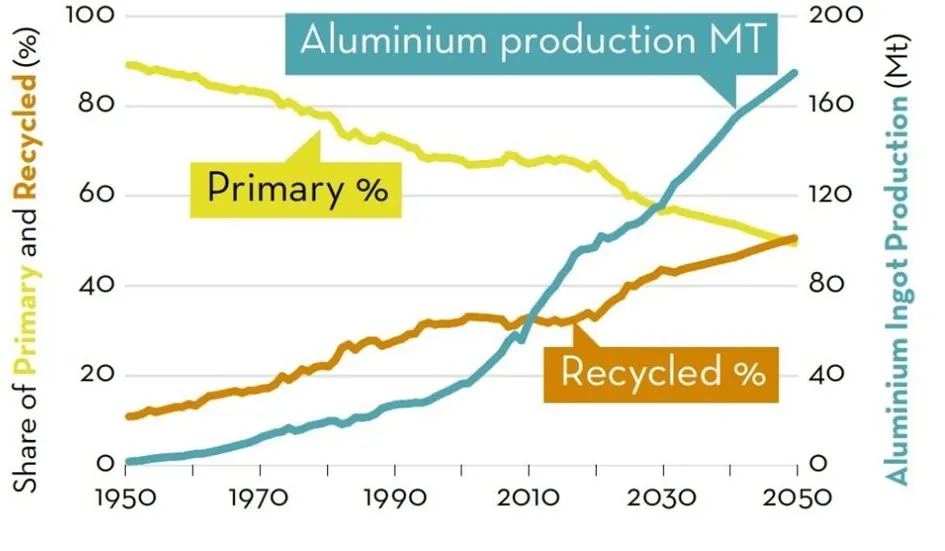
Although primary aluminum is declining, it will still account for 50% of the aluminum produced in 2050. Therefore, primary aluminum needs to be decarbonized as much as possible. Looking at the main drivers of emissions, there are four segments that need to be addressed:
- The carbon anode and its production
- The production of aluminium oxide from Bauxite in the Bayer process
- The electrolysis or Hall-Héroult process
- Processing and casting of aluminium
Clean electricity and storage to decarbonize process heat
Out of these, the biggest energy share has the Hall-Héroult process using large amounts of electricity with a lot of Scope 2 emissions. Switching to renewable sources already takes down a heavy weight. The anodes are made from carbon. In the electrolytic process they bind the oxygen from the aluminium oxide to carbon and release greenhouse gases.
While there are discussions about using different materials for the anode and other processes, these remain inconclusive and it is questionable whether they will reduce emissions due to the high energy requirements. Therefore, the best option for reducing emissions from the anode part is to switch to renewable heat in anode production and increase energy efficiency. The baking of anodes is done by up to 1,200 °C in long cycles. Before analyzing the waste heat options, we continue with the electrification options, especially for the decarbonization of the Bayer process.
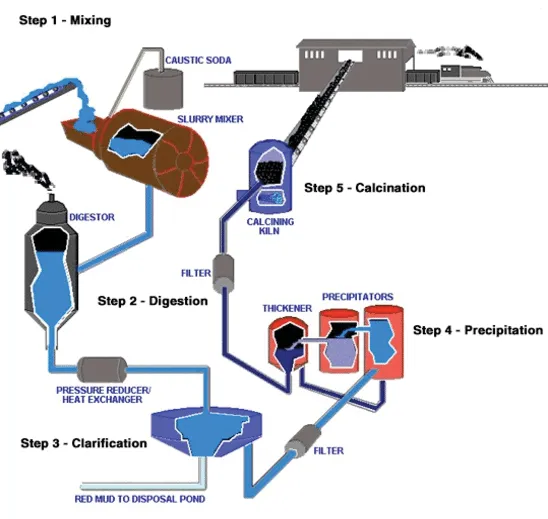
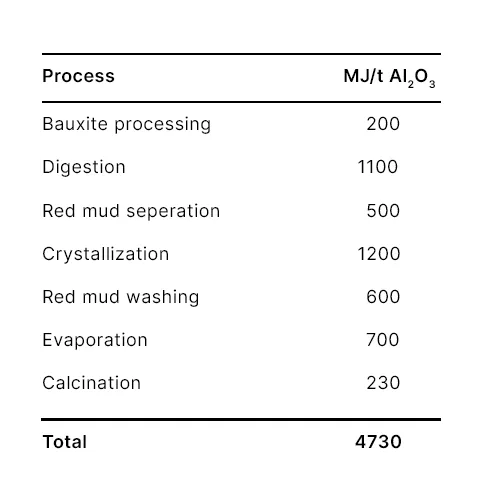
This process uses digesters and calcination to convert bauxite to aluminum oxide. The digesters use steam at 110 °C - 280 °C depending on the mineral. This can be provided by Kraftblock's Net-Zero Heat System using flexible green power. In addition to digestion, evaporation and product washing the calcination is an energy intensive part of alumina production. Calcination of the alumina takes place at 800 °C - 1,200 °C. Pre-heating the furnaces, the gas and possibly even replacing fossil fuel entirely can also be done by the combination of power-to-heat and high-temperature storage. Fluidized bed furnaces can reduce the energy amount almost 40% (Qinkertz, 2002).
When aluminium is casted or remelted in the secondary production route, heat up to 900 °C is used in the different furnace types. Aluminium melts at 660°C. This means, electrification and hot air can most likely be integrated into existing furnaces and replace fossil fuels with the Kraftblock systems.
Boosting energy efficiency with waste heat
Aluminum production also generates a lot of waste heat, leading to projects to use the heat for district heating or to produce malt for brewing. But recovering waste heat from aluminum production is not easy. Especially for in-house applications. This is due to the fact that many parts of the production process are separated from each other. For example, the Bayer process is often on a different plant than the electrolytic process. Also, many steps are batch processes, making it difficult to use heat directly in other processes.
This means that waste heat has to be transferred. For the most energy-intensive process, electrolysis, waste heat recovery is difficult. Considerable heat radiates from the surface and dissipates quickly. Until now, the only way to capture this heat was to use a sidewall heat exchanger. There is also heat that leaves the furnace when it is opened and the flow is collected by several ducts that carry the gas to the treatment. The gas is between 100°C and 120°C, and a plant using the Hall-Héroult process could potentially provide district heating for a small city (Nowicki/Gosselin,2012).
In the Bayer process, several processes are heat driven, leaving a lot of waste heat. Typically, some of this is already being used, such as the exhaust gases from the calcination furnace for dehydration of aluminum hydroxide.
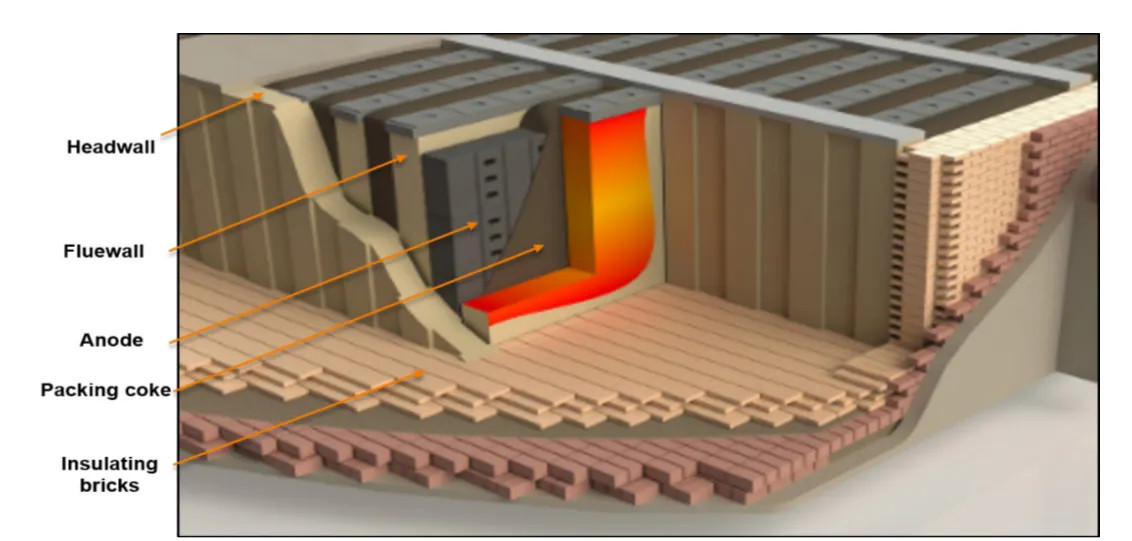
Anodes are baked in furnaces over a long period of time using coke. In the reference plant studied by Noicki and Gosselin, about 5 MW can be found continuously in the waste gas from the baking furnaces, which corresponds to 44 GWh per year and a temperature close to 300 °C. There could also be a good use case for waste heat in the anode cooling sections, where temperatures can reach high levels depending on the furnace section, and where the heat flux from the furnace surfaces can reach 1,100 °C. More research is needed to find a way to recover this heat.
Casting can also generate significant waste heat in excess of 700 °C, as aluminum is often heated to higher temperatures and then allowed to cool before casting can begin. However, it is difficult to capture this waste heat in open furnaces. During casting, gas is burned to keep temperatures high, so there is an intermittent stream of waste heat in the stack system that is easily accessible. There is also a significant amount of waste heat in the water that cools the furnaces and castings; 43 GWh was lost annually in the reference plants.
In addition to various district and space heating applications, the higher quality waste heat can be used to preheat castings or dry coke. If scrap or alloys are used, they can also be preheated. The anode and cathode can also be preheated and an ORC can generate electricity if no other use is found.
References
Guminski, A., Rouyrre, E., & Wiener, M. C. (FfE). (2019). CO₂-Verminderung in der Primäraluminiumherstellung. https://www.ffe.de/wp-content/uploads/2021/08/CO2-Verminderung_in_der_Primaeraluminium.pdf
International Aluminium Institute (IAI). (2021). IAI Material Flow Model – 2021 Update. Online: https://international-aluminium.org/resource/iai-material-flow-model-2021-update/
IEA. (2023). Tracking Aluminium. https://www.iea.org/energy-system/industry/aluminium
Lightmetalage.com. (2020). IAI Releases 2020 Total Global Primary Aluminum and Alumina Production Data. Online: https://www.lightmetalage.com/news/industry-news/smelting/iai-releases-2020-total-global-primary-aluminum-and-alumina-production-data/
Nowicki, C., & Gosselin, L. (2012). An Overview of Opportunities for Waste Heat Recovery and Thermal Integration in the Primary Aluminum Industry. JOM, 64, 990–996. https://doi.org/10.1007/s11837-012-0367-4
Qinertz, R. (2002). Optimierung der Energienutzung bei der Aluminiumherstellung. Dissertation, RWTH Aachen. Online: https://publications.rwth-aachen.de/record/52846/files/Quinkertz_Rainer.pdf
Tabereaux, A. (2010). Hungarian red mud disaster: Addressing environmental liabilities of alumina residue storage & disposal. Light Metal Age, 68, 22–24.
Zaidani, M., Abu Al-Rub, R., Tajik, A. R., & Shamim, T. (2016). Effects of Flue Wall Deformation on Aluminum Anode Baking Homogeneity and Temperature Distribution. 34th International Conference and Exhibition of ICSOBA. Online: https://icsoba.org/proceedings/34th-conference-and-exhibition-icsoba-2016/?doc=39
